September 12, 2024
Sinclair and Lanluma® is proud to announce that it has had a new study published in The Journal of Cosmetic Dermatology in September 2024
Safety and effectiveness results of an innovative injectable poly-L-lactic acid-based collagen stimulator (Lanluma®)—Clinical outcomes at 9 months in a post-market study
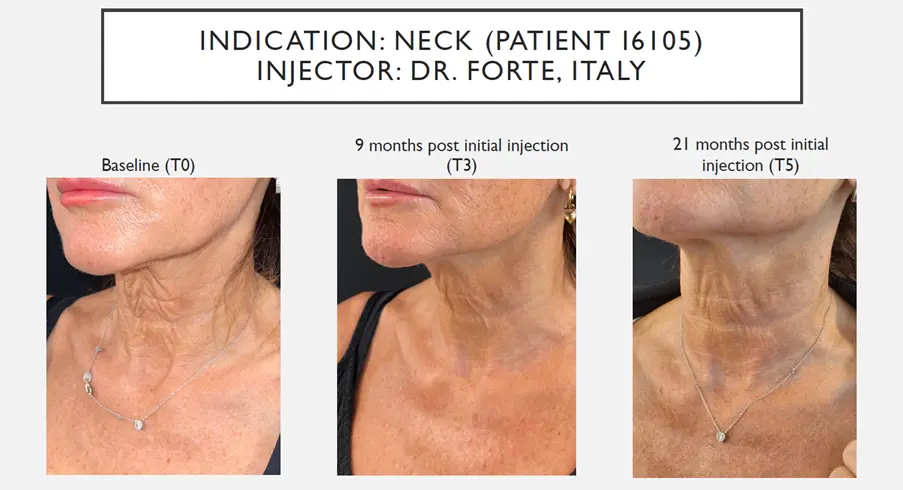
The study was carried out in six European clinics (Greece, UK, Italy, Spain and two in Austria) targeting female and male participants aged 40-65 years. Seventy participants had 99 treatment sessions and represents a representative sample to consider the use of Lanluma in these procedures. The study was carried out to demonstrate the safety and efficacy of Lanluma in five body procedures: neck (31%), upper arm (20%), hand (17%) thigh (16%) and décolleté(15%). The analysis is based on the clinical outcomes data (safety and effectiveness) collected from investigators and participants between the first injection (T0, September 2022) and 9 months thereafter (T3, June 2023) in the treatment of five body-contouring areas.
Both investigators and participants reported high levels of satisfaction during the nine-month follow-up period with the treatments in five body areas. Satisfaction was particularly high among participants at the third visit of Neck procedures rated 87.1% with overall appearance, 93.5% satisfaction with skin smoothness, 90.3% satisfaction with skin texture and thickness and 93.6% satisfied with the improvement in the look of the skin. While for Décolleté procedures 93.3% of participants were satisfied with the overall look of their skin, improved skin texture and reduced wrinkles.
Lead investigator, Dr Moises Amselem noted that “These positive clinical outcomes can be attributed to the correct implementation of best practices and recommendations, and the rheological properties of Lanluma.”
Sinclair continues to demonstrate that Lanluma can achieve, through studies, excellent outcomes with high levels of satisfaction from both participants and practitioners and offers further confidence in Lanluma, but also combined with skilled injectors following detailed protocols, delivers outstanding results.
Sinclair would like to extend their immense thanks to all the investigators who contributed to this study for their professionalism and commitment to developing the scientific platform for Lanluma and their continuing support to complete the study.
UKBR-00851069-001-000